- [email protected]
- 27-31 Wright St, Clayton VIC 3168
- Contact
Blog Details
-
EZADEH > My research > CRISPR/Cas for Diagnosis and Detection
04Mar
CRISPR/Cas for Diagnosis and Detection
by Elazadeh, 0 Comments

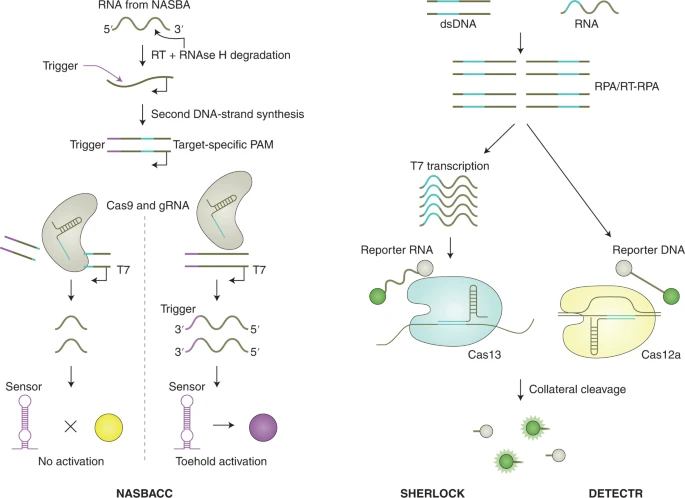
CRISPR is being used as a diagnostic tool for detecting nucleic acids involved in both infectious and non-infectious diseases! CRISPR–Cas systems can be divided, according to evolutionary relationships, into two classes, six types and several subtypes. The classes of CRISPR–Cas system are defined by the nature of the ribonucleoprotein effector complex: class 1 systems are characterized by a complex of multiple effector proteins, and class 2 systems encompass a single crRNA-binding protein. The design of crRNAs for the different effector proteins used in CRISPR diagnostics follows the same principles as those of other CRISPR applications. Among the diverse CRISPR systems, class 2 systems have primarily been applied for diagnostics, as these systems are simpler to reconstitute. They include enzymes with collateral activity, which serve as the backbone of many CRISPR-based diagnostic assays. Class 1 systems (such as the type III effector nuclease Csm6 or Cas10) have also been engineered for diagnostics, either in combination with components of the class 2 system or with the native type III complex.
The first CRISPR-based diagnostic methods that were developed largely used Cas9 variants, which recognize double-stranded DNA (dsDNA). The main principles of the many different Cas9-based approaches for sensing DNA that have been reported include guide-directed reconstitution of split proteins by catalytically inactive Cas9 partners, Cas9-based destruction of PAM-containing sites and Cas9-induced unwinding of the non-targeted DNA strand as a targeting site for isothermal amplification.
Over the past 40 years, there have been recurrent large-scale epidemics from emerging viruses such as HIV, SARS and Middle East respiratory syndrome coronaviruses, 2009 pandemic influenza H1N1 virus, Ebola virus, Zika virus and most recently SARS-CoV-2. All of these epidemics most likely resulted from an initial zoonotic animal-to-human transmission event, with either clinically apparent or occult spread into vulnerable human populations. Each time, a lack of rapid, accessible and accurate molecular diagnostic testing has hindered the public health response to the emerging viral threat. In early January 2020, a cluster of cases of pneumonia from a new coronavirus, SARS-CoV-2 (with the disease referred to as COVID-19), was reported in Wuhan, China. This outbreak has spread rapidly, with over 1.2 million reported cases and 64,500 deaths worldwide as of 4 April 2020. Person-to-person transmission from infected individuals with no or mild symptoms has been reported. Assays using quantitative RT–PCR (qRT–PCR) approaches for detection of the virus in 4–6 h have been developed by several laboratories, including an emergency use authorization (EUA)-approved assay developed by the US Centers for Disease Control and Prevention.

-
Tags:
- Laboratory techniques
