- [email protected]
- 27-31 Wright St, Clayton VIC 3168
- Contact
01Aug
New insights into fruit fly cell regulation may offer clues for treating brain tumors
by Elazadeh, 0 Comments

Working model of the termination of medulla NBs. Credit: eLife (2024). DOI: 10.7554/eLife.96876
Peter Mac researchers have discovered new insights into neural stem cell development in fruit flies that may provide answers on how brain tumors grow in humans.
Led by Associate Professor Louise Cheng and Ph.D. candidate Phuong-Khanh Nguyen, the study published in eLife focused on understanding how fruit fly neural stem cells, called neuroblasts, stop dividing in the fly’s visual processing center called the optic lobes.
Neural stem cells are found in the brain and spinal cord and they have the ability to replicate themselves, differentiate into other types of brain cells and aid in repairing parts of the brain.
Stem cells should stop making new cells once enough neurons are made during development.
However, sometimes this process goes wrong, and cells keep dividing when they should have stopped. This out-of-control proliferation is similar to how cancer arises in humans.
Associate Professor Cheng said their findings provide answers to how and when neuroblasts in fruit flies cease proliferation. This lays the foundation for further investigation into how brain cancer—which has a five-year survival rate of just 23 percent—arises in the human brain.
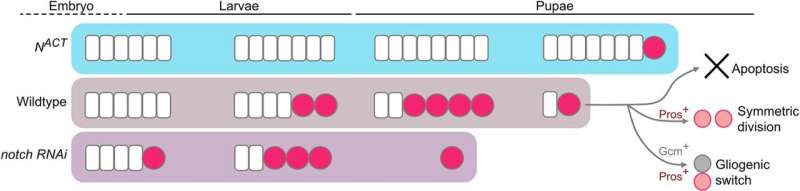
“Our study revealed neuroblasts drastically decrease 12–18 hours after the larvae transition into pupae, disappearing completely by 30 hours into pupae life,” she said.
“These findings show that the timing of neuroblast disappearance is influenced by neuroepithelium cells, the pool of stem cells that generate neuroblasts during the early stages of development.”
The research discovered that speeding up the transition of neuroblasts from the neuroepithelium led to their quicker disappearance from the optic lobe. Conversely, delaying this transition allowed neuroblasts to persist for a longer period. This timing of neuroblast production plays a crucial role in neuroblast termination.
Further analysis showed that neuroblasts are lost through several mechanisms, including programmed cell death (apoptosis), division into mature neurons, or conversion into glial cells.
“Understanding these processes gives us a clearer picture of how neuroblasts are regulated and how they transition into different cell types,” Cheng said.
“This helps us to map out how normal human brain development works, which is essential for understanding why and how brain tumors arise.
“If neuroblasts and their equivalent stem cells don’t know when to stop dividing, it can lead to uncontrolled growth, resulting in brain tumors. By understanding how these cells stop proliferating during normal development, we can better understand how brain cancers develop and potentially find new ways to treat them.”
Associate Professor Cheng and Nguyen’s ongoing research aims to explore how mutations affecting brain cells can have different outcomes depending on their location in the brain. This could help explain why some brain regions are more prone to tumors than others.
Additionally, with brain tumors accounting for approximately 25% of children’s cancers compared to just 2% of adult cancers, Associate Professor Cheng explained that understanding how brain development is regulated could shed light on why many children are affected by brain tumors.
More information:
Phuong-Khanh Nguyen et al, Drosophila medulla neuroblast termination via apoptosis, differentiation, and gliogenic switch is scheduled by the depletion of the neuroepithelial stem cell pool, eLife (2024). DOI: 10.7554/eLife.96876
Journal information:
eLife
