- [email protected]
- 27-31 Wright St, Clayton VIC 3168
- Contact
Blog Details
-
EZADEH > My research > Cord Blood-Derived MSCs in Neuroregeneration
09May
Cord Blood-Derived MSCs in Neuroregeneration
by Elazadeh, 0 Comments
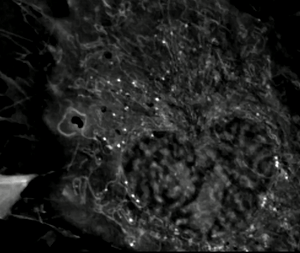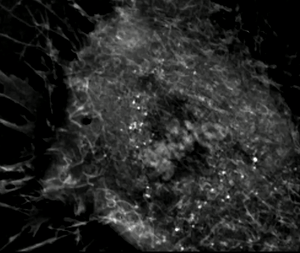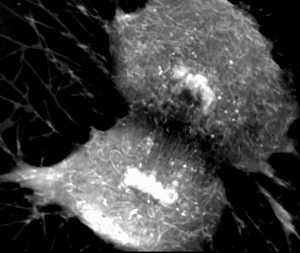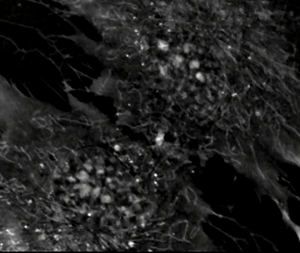
Exploring the Frontier of Neuroregeneration with Mesenchymal Stem Cells
Neuroregeneration, the intricate process of restoring damaged or lost neurons in the nervous system, represents an exciting frontier for scientific exploration. Among the emerging strategies, the use of mesenchymal stem cells (MSCs) holds immense promise. MSCs, derived from diverse sources like bone marrow, adipose tissue, and cord blood, are a type of adult stem cell known for their versatility. They can differentiate into various cell types, including neurons and supportive cells within the nervous system. What makes MSCs particularly fascinating for neuroregenerative therapies are their unique attributes. In addition to their ability to self-renew and expand, MSCs exhibit paracrine effects by secreting a multitude of bioactive molecules. These secreted factors, including growth factors and cytokines, facilitate complex intercellular communication, promoting tissue repair, regulating inflammation, and triggering processes crucial for neuroregeneration. Both preclinical and clinical studies have unveiled the remarkable potential of MSCs in neuroregeneration.
Transplanting MSCs into injured or diseased neural tissues has shown that these cells can adopt neuronal-like characteristics and integrate into existing neural networks. Moreover, the paracrine effects of MSCs enhance endogenous neural stem cell activity, stimulate neuroplasticity, promote neuronal survival, and modulate the immune response, creating a conducive microenvironment for neuroregeneration. Among the various sources of MSCs, cord blood-derived MSCs offer an intriguing avenue for research. Cord blood, obtained from the umbilical cord and placenta, is a rich source of stem cells with vast potential. Cord blood-derived MSCs exhibit similar properties to those from other sources, such as bone marrow and adipose tissue. They share key traits, including multipotency, self-renewal capabilities, and immunomodulatory properties, underlining their suitability for neuroregenerative applications. Beyond their regenerative and paracrine properties, MSCs possess remarkable immunomodulatory abilities. Through the secretion of diverse factors, MSCs can modulate the complex interplay between immune cells in damaged neural tissue. This immunomodulatory prowess helps shape the immune response favorably for neuroregeneration.
While the application of MSCs in neuroregeneration captures scientific interest, several exciting challenges and considerations must be addressed. These include determining optimal administration routes, timing of transplantation, cell survival, integration into host tissue, and long-term safety profiles. Additionally, unraveling the intricate molecular mechanisms underlying the therapeutic effects of MSCs in neuroregeneration continues to intrigue researchers. The use of mesenchymal stem cells, including those derived from cord blood, offers a captivating avenue for neuroregeneration. Their multipotency, paracrine effects, and immunomodulatory capabilities intertwine to create a mesmerizing landscape of cellular interactions, providing a platform for neuronal repair and functional recovery. Continued research into the intricate molecular mechanisms of MSC-based therapies promises novel scientific discoveries and significant advancements in the field of neuroregeneration.
The Precise Procedure of Cord Blood Stem Cell Extraction and Its Promising Potential
Cord blood stem cells, a valuable resource for regenerative medicine, are extracted through a meticulous procedure known as cord blood collection or cord blood banking. When a baby is born, the healthcare provider clamps and cuts the umbilical cord before delivering the placenta. This moment is critical for collecting the precious cord blood.
Using a sterile collection kit, the healthcare provider inserts a needle into the umbilical vein of the clamped cord, allowing the cord blood to flow naturally from the placenta into a collection bag or syringe. Once collected, the cord blood, along with all necessary documentation and labeling, is transported to the laboratory. In the lab, the cord blood undergoes processing to extract the stem cells. This typically involves centrifugation, which separates the stem cells from other components like red blood cells and plasma. This step ensures that the stem cells are isolated and concentrated for future use.
After processing, the stem cells are cryopreserved for long-term storage. Cryopreservation involves freezing the stem cells at very low temperatures and storing them in specialized containers designed to maintain their viability over extended periods. This process allows the stem cells to be stored for many years while preserving their regenerative potential. The extracted cord blood stem cells can be stored in either a private cord blood bank or a public cord blood bank. Private banks store the cord blood exclusively for the family’s use, providing a potential source of stem cells for the baby or their family members in the future. Public banks, on the other hand, store the cord blood for potential use by anyone in need of a stem cell transplant, offering a valuable resource for individuals requiring a stem cell match. In summary, cord blood extraction is a carefully orchestrated process that ensures the successful collection and preservation of valuable stem cells from the umbilical cord. This procedure offers families the opportunity to harness the regenerative potential of these stem cells for potential future use in medical treatments and therapies.
