- [email protected]
- 27-31 Wright St, Clayton VIC 3168
- Contact
Blog Details
-
EZADEH > My research > Area postrema and Parkinson’s disease
15Mar
Area postrema and Parkinson’s disease
by Elazadeh, 0 Comments
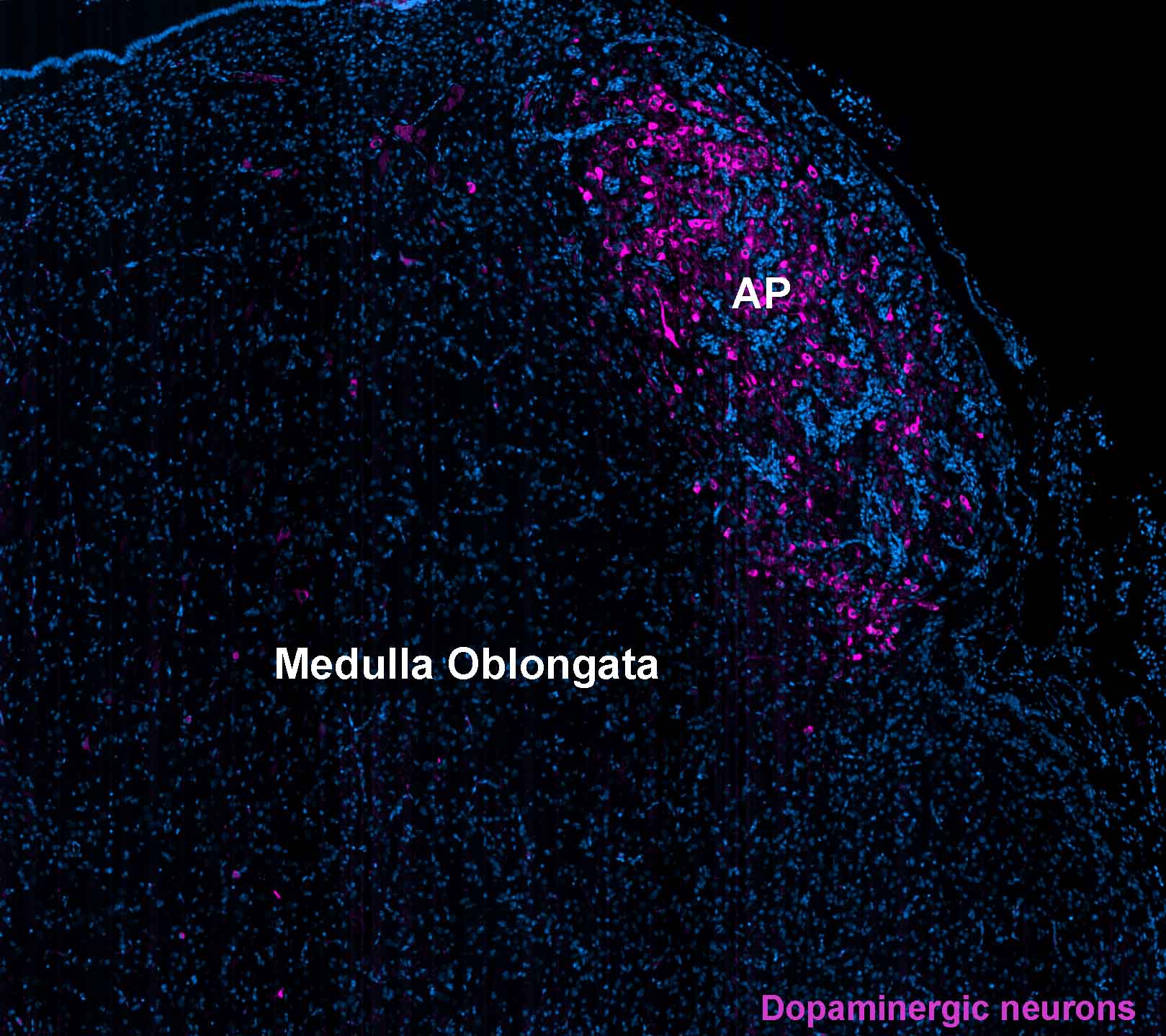
Parkinson’s disease is a complex and debilitating neurodegenerative disorder that is characterized by the loss of dopaminergic neurons in the substantia nigra region of the brain. While this is the most well-known aspect of the disease, recent research has identified the involvement of other brain regions in the pathology of Parkinson’s disease, including the area postrema. The area postrema is a small structure located in the brainstem that plays an important role in regulating various physiological functions, including the control of vomiting. In this article, we will discuss the current understanding of the involvement of the area postrema in Parkinson’s disease, including its role in the development of non-motor symptoms and its potential as a therapeutic target.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that affects the nervous system, particularly the motor system. The pathological hallmark of PD is the progressive loss of dopamine-producing neurons in the substantia nigra pars compacta (SNc), resulting in dopamine depletion in the striatum. However, other brain regions are also affected in PD, including the area postrema (AP), a small structure located in the medulla oblongata, which plays an essential role in the control of autonomic and visceral functions. The AP has been implicated in the pathophysiology of PD due to its connections with the dopaminergic and noradrenergic systems, as well as its role in the regulation of nausea and vomiting, which are common non-motor symptoms of PD. This paper aims to review the literature on the involvement of the AP in PD and discuss its potential implications for the diagnosis and treatment of this disorder.
The AP is a small brain structure located in the dorsal medulla oblongata, adjacent to the fourth ventricle, and outside the blood-brain barrier. The AP is an essential component of the area postrema/nucleus tractus solitarii complex, which plays a critical role in the regulation of autonomic and visceral functions, such as blood pressure, heart rate, gastrointestinal motility, and vomiting (1). The AP is richly innervated by afferent fibers that convey information from the periphery to the brainstem, including the gastrointestinal tract, liver, and spleen. The AP also receives projections from the dopaminergic and noradrenergic systems, which regulate its activity (2).
One of the hallmarks of PD is the loss of dopaminergic neurons in the SNc, resulting in dopamine depletion in the striatum, which is responsible for the motor symptoms of the disease. However, dopamine depletion also occurs in other brain regions, including the AP, where it can affect the regulation of autonomic and visceral functions. Several studies have shown that dopamine receptor agonists can modulate the activity of the AP and improve the symptoms of nausea and vomiting in PD patients (3, 4). This suggests that dopamine depletion in the AP may contribute to the development of non-motor symptoms in PD.
In addition to its connections with the dopaminergic system, the AP also receives projections from the noradrenergic system, which regulates its activity. Noradrenaline is involved in the modulation of the baroreceptor reflex, which regulates blood pressure, and the chemoreceptor reflex, which regulates ventilation in response to changes in the blood gas levels. Studies have shown that noradrenaline depletion in the AP can impair the baroreceptor and chemoreceptor reflexes and contribute to the development of orthostatic hypotension and breathing difficulties, which are common non-motor symptoms of PD (5, 6).
Furthermore, the AP is also involved in the regulation of the immune system, which has been implicated in the pathophysiology of PD. The AP is an essential site of interaction between the immune system and the central nervous system, where it regulates the migration of immune cells and the release of cytokines in response to peripheral inflammation. Studies have shown that inflammation can activate microglia in the AP and contribute to the development of neuroinflammation and neurodegeneration in PD (7, 8).
Finally, the AP is also involved in the regulation of the gut-brain axis, which has been implicated in the pathophysiology of PD. The gut-brain axis refers to the bidirectional communication between the gastrointestinal tract and the central nervous system, where it regulates the modulation of the gut microbiota, the secretion of gut hormones, and the activation of the enteric nervous system.
References:
1. Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262(4):546-62.
2. Robertson, Alan S. Disorders of the autonomic nervous system. Routledge, 2019.
3. Chinta, Shankar J., and Julie K. Andersen. “Dopaminergic neurons.” The international journal of biochemistry & cell biology 37.5 (2005): 942-946.
4. Arias-Carrión, Óscar, and Ernst Pöppel. “Dopamine, learning, and reward-seeking behavior.” Acta neurobiologiae experimentalis 67.4 (2007): 481-488.
5. Raymon, Lionel P., and H. Chip Walls. “Pharmacology of cannabinoids.” Marijuana and the Cannabinoids (2007): 97-123.
6. Sclocco, Roberta, et al. “Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI.” Neuroimage 168 (2018): 412-426.
7. Goehler, L. E., A. Erisir, and R. P. A. Gaykema. “Neural–immune interface in the rat area postrema.” Neuroscience 140.4 (2006): 1415-1434.
8. Vargas-Caraveo, Alejandra, David Guillermo Pérez-Ishiwara, and Alejandro Martínez-Martínez. “Chronic psychological distress as an inducer of microglial activation and leukocyte recruitment into the area postrema.” Neuroimmunomodulation 22.5 (2015): 311-321.
