- [email protected]
- 27-31 Wright St, Clayton VIC 3168
- Contact
23Aug
A new culprit in Huntington’s: Brain organoid model implicates gene in disease progression
by Elazadeh, 0 Comments
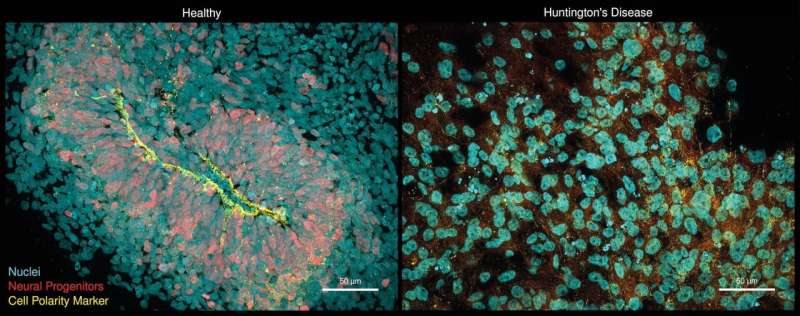

Huntington’s Disease organoids (right) exhibit almost no neural progenitors (red) and also show defects in cellular polarity (yelllow). These defects have been described in the literature in human fetuses with Huntington’s disease. Credit: Selene Lickefett, Heinrich-Heine-Universität Düsseldorf, Werner Dykstra, Max Delbrück Center
For the first time, researchers have implicated the gene CHCHD2 in Huntington’s disease (HD)—an incurable genetic neurodegenerative disorder—and identified the gene as a potentially new therapeutic target. In a brain organoid model of the disease, the researchers found that mutations in the Huntington gene HTT also affect CHCHD2, which is involved in maintaining the normal function of mitochondria.
The study was published in Nature Communications.
Six different labs at the Max Delbrück Center participated in the study, led by Dr. Jakob Metzger of the Quantitative Stem Cell Biology lab at the and the Stem Cell Metabolism lab of Professor Alessandro Prigione at Heinrich Heine University Düsseldorf (HHU). Each lab contributed their unique expertise on Huntington’s disease, brain organoids, stem cell research and genome editing.
“We were surprised to find that Huntington’s disease can impair early brain development through defects associated with mitochondrial dysfunction,” says Dr. Pawel Lisowski, co-lead author in the Metzger lab at the Max Delbrück Center.
Moreover, “the organoid model suggests that HTT mutations damage brain development even before clinical symptoms appear, highlighting the importance of detecting the late-onset neurodegenerative disease early,” Selene Lickfett, co-lead author and a doctoral student in the Faculty of Mathematics and Natural Science in the lab of Prigione at HHU adds.
The unusual repetition of three letters
An organoid is a three-dimensional, organ-like structure that researchers grow in a laboratory from stem cells. Depending on the disease and research question, organoids can be grown from different types of tissue. Only a few millimeters in size, they serve as a model for how different cell types interact. No other bench-top model provides such a detailed look at the function of cells in the human body.
Huntington’s disease is caused when the nucleotides cytosine, adenine and guanine are repeated an excessive number of times in the Huntington gene HTT. People with 35 or fewer repeats are generally not at risk of developing the disease, while carrying 36 or more repeats has been associated with disease. The greater the number of repeats, the earlier the disease symptoms are likely to appear, explains Metzger, a senior author of the study. The mutations cause nerve cells in the brain to progressively die.
Those affected steadily lose muscle control and develop psychiatric symptoms such as impulsiveness, delusions and hallucinations. Huntington’s disease affects approximately five to 10 in every 100,000 people worldwide. Existing therapies only treat the symptoms of the disease, they don’t slow its progression or cure it.
The challenge of HTT gene editing
To study how mutations in the HTT gene affect early brain development, Lisowski first used variants of the Cas9 gene editing technology and manipulation of DNA repair pathways to modify healthy induced pluripotent stem cells such that they carry a large number of CAG repeats. This was technically challenging because gene editing tools are not efficient in gene regions that contain sequence repeats, such as the CAG repeats in HTT, says Lisowski.
The genetically modified stem cells were then grown into brain organoids—three-dimensional structures that resemble early-stage human brains. When the researchers analyzed gene expression profiles of the organoids at different stages of development, they noticed that the CHCHD2 gene was consistently under expressed, which reduced metabolism of neuronal cells.
CHCHD2 is involved in ensuring the health of mitochondria—the energy producing structures in cells. CHCHD2 has been implicated in Parkinson’s disease, but never before in Huntington’s.
They also found that when they restored the function of the CHCHD2 gene, they could reverse the effect on neuronal cells. “That was surprising,” says Lickfett. “It suggests in principle that this gene could be a target for future therapies.”
Moreover, defects in neural progenitor cells and brain organoids occurred before potentially toxic aggregates of mutated Huntingtin protein had developed, adds Metzger, indicating that disease pathology in the brain may begin long before it is clinically evident.
“The prevalent view is that the disease progresses as a degeneration of mature neurons,” says Prigione. “But if changes in the brain already develop early in life, then therapeutic strategies may have to focus on much earlier time-points.”
Wide-reaching implications
“Our genome editing strategies, in particular the removal of the CAG repeat region in the Huntington gene, showed great promise in reversing some observed developmental defects. This suggests a potential gene therapy approach,” says Prigione. Another potential approach could be therapies to increase CHCHD2 gene expression, he adds.
The findings may also have broader applications for other neurodegenerative diseases, Prigione adds. “Early treatments that reverse the mitochondrial phenotypes shown here could be a promising avenue for counteracting age-related diseases like Huntington’s disease.”
More information:
Lisowski, P. et al. Mutant Huntingtin impairs neurodevelopment in human brain organoids through CHCHD2-mediated neurometabolic failure, Nature Communications (2024). DOI: 10.1038/s41467-024-51216-w. www.nature.com/articles/s41467-024-51216-w
Journal information:
Nature Communications
