- [email protected]
- 27-31 Wright St, Clayton VIC 3168
- Contact
13Jun
Target detection increases pupil diameter and enhances memory for background scenes during multi-tasking – Scientific Reports
by Elazadeh, 0 Comments
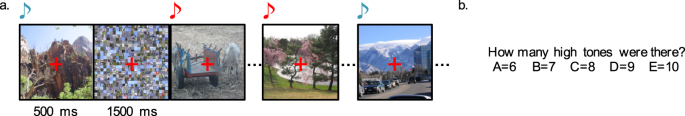
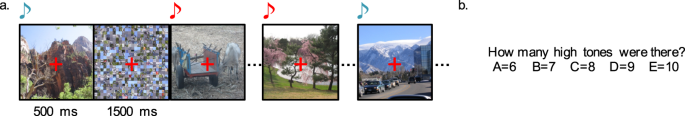
-
Kinchla, R. A. Attention. Annu. Rev. Psychol. 43, 711–742 (1992).
CAS
ArticleGoogle Scholar
-
Duncan, J. The locus of interference in the perception of simultaneous stimuli. Psychol. Rev. 87, 272–300 (1980).
CAS
ArticleGoogle Scholar
-
Lin, J. Y., Pype, A. D., Murray, S. O. & Boynton, G. M. Enhanced Memory for Scenes Presented at Behaviorally Relevant Points in Time. PLoS Biol 8, e1000337 (2010).
Article
Google Scholar
-
Swallow, K. M. & Jiang, Y. V. The Attentional Boost Effect: Transient increases in attention to one task enhance performance in a second task. Cognition 115, 118–132 (2010).
Article
Google Scholar
-
Turker, H. B. & Swallow, K. M. Attending to behaviorally relevant moments enhances incidental relational memory. Mem. Cognit. 1–16, https://doi.org/10.3758/s13421-018-0846-0 (2018).
-
Swallow, K. M., Makovski, T. & Jiang, Y. V. The Selection of Events in Time Enhances Activity Throughout Early Visual Cortex. J. Neurophysiol. https://doi.org/10.1152/jn.00472.2012 (2012).
-
Makovski, T., Swallow, K. M. & Jiang, Y. V. Attending to unrelated targets boosts short-term memory for color arrays. Neuropsychologia 49, 1498–1505 (2011).
Article
Google Scholar
-
Swallow, K. M. & Jiang, Y. V. Perceptual load and attentional boost: A study of their interaction. J. Exp. Psychol. Hum. Percept. Perform. 40, 1034–1045 (2014).
Article
Google Scholar
-
Spataro, P., Mulligan, N. W. & Rossi-Arnaud, C. Divided Attention Can Enhance Memory Encoding: The Attentional Boost Effect in Implicit Memory. J. Exp. Psychol. Learn. Mem. Cogn. 1223–1231, https://doi.org/10.1037/a0030907 (2013).
-
Mulligan, N. W., Spataro, P. & Picklesimer, M. The attentional boost effect with verbal materials. J. Exp. Psychol. Learn. Mem. Cogn. 40, 1049–1063 (2014).
Article
Google Scholar
-
Swallow, K. M. & Atir, S. The role of value in the attentional boost effect. Q. J. Exp. Psychol. 1747021818760791, https://doi.org/10.1177/1747021818760791 (2018).
-
Mulligan, N. W. & Spataro, P. Divided Attention Can Enhance Early-Phase Memory Encoding: The Attentional Boost Effect and Study Trial Duration. J. Exp. Psychol. Learn. Mem. Cogn. https://doi.org/10.1037/xlm0000055 (2014).
-
Spataro, P., Mulligan, N. W. & Rossi-Arnaud, C. Limits to the attentional boost effect: the moderating influence of orthographic distinctiveness. Psychon. Bull. Rev. 1–6, https://doi.org/10.3758/s13423-014-0767-2 (2014).
-
Swallow, K. M. & Jiang, Y. V. Attentional Load and Attentional Boost: A Review of Data and Theory. Front. Psychol. 4 (2013).
-
Smith, S. A. & Mulligan, N. W. Distinctiveness and the attentional boost effect. J. Exp. Psychol. Learn. Mem. Cogn. 44, 1464–1473 (2018).
Article
Google Scholar
-
Swallow, K. M. & Jiang, Y. V. The attentional boost effect really is a boost: Evidence from a new baseline. Atten. Percept. Psychophys. 1–10, https://doi.org/10.3758/s13414-014-0677-4 (2014).
-
Swallow, K. M. & Jiang, Y. V. Goal-Relevant Events Need Not be Rare to Boost Memory for Concurrent Images. Atten. Percept. Psychophys. 74, 70–82 (2012).
Article
Google Scholar
-
Swallow, K. M. & Jiang, Y. V. The role of timing in the attentional boost effect. Atten. Percept. Psychophys. 73, 389–404 (2011).
Article
Google Scholar
-
Corbetta, M., Patel, G. & Shulman, G. L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 58, 306–324 (2008).
CAS
ArticleGoogle Scholar
-
Serences, J. T. et al. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol. Sci. 16, 114–122 (2005).
Article
Google Scholar
-
Jack, A. I., Shulman, G. L., Snyder, A. Z., McAvoy, M. P. & Corbetta, M. Separate modulations of human V1 associated with spatial attention and task structure. Neuron 51, 135–147 (2006).
CAS
ArticleGoogle Scholar
-
Sokolov, E. N., Nezlina, N. I., Polyanskii, V. B. & Evtikhin, D. V. The orientating reflex: The ‘targeting reaction’ and ‘searchlight of attention’. Neurosci. Behav. Physiol. 32, 347–362 (2002).
CAS
ArticleGoogle Scholar
-
Nieuwenhuis, S., Geus, E. J. D. & Aston‐Jones, G. The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology 48, 162–175 (2011).
Article
Google Scholar
-
Sara, S. J. & Bouret, S. Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron 76, 130–141 (2012).
CAS
ArticleGoogle Scholar
-
Wang, C.-A. & Munoz, D. P. A circuit for pupil orienting responses: implications for cognitive modulation of pupil size. Curr. Opin. Neurobiol. 33, 134–140 (2015).
CAS
ArticleGoogle Scholar
-
Steiner, G. Z. & Barry, R. J. Pupillary responses and event-related potentials as indices of the orienting reflex: Pupillary responses and ERPs as indices of the OR. Psychophysiology 48, 1648–1655 (2011).
Article
Google Scholar
-
Kamp, S.-M. & Donchin, E. ERP and pupil responses to deviance in an oddball paradigm: ERP and pupil responses in an oddball paradigm. Psychophysiology 52, 460–471 (2015).
Article
Google Scholar
-
Aston-Jones, G. & Cohen, J. D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
CAS
ArticleGoogle Scholar
-
Nieuwenhuis, S., Gilzenrat, M. S., Holmes, B. D. & Cohen, J. D. The role of the locus coeruleus in mediating the attentional blink: A neurocomputational theory. J. Exp. Psychol. Gen. 134, 291–307 (2005).
Article
Google Scholar
-
Gilzenrat, M. S., Nieuwenhuis, S., Jepma, M. & Cohen, J. D. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect. Behav. Neurosci. 10, 252–269 (2010).
Article
Google Scholar
-
Unsworth, N. & Robison, M. K. A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychon. Bull. Rev. 24, 1282–1311 (2017).
Article
Google Scholar
-
Brink, R. L., van den, Murphy, P. R. & Nieuwenhuis, S. Pupil Diameter Tracks Lapses of Attention. PLOS ONE 11, e0165274 (2016).
Article
Google Scholar
-
Aston-Jones, G., Rajkowski, J., Kubiak, P. & Alexinsky, T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 14, 4467–4480 (1994).
CAS
ArticleGoogle Scholar
-
Rajkowski, J., Majczynski, H., Clayton, E. & Aston-Jones, G. Activation of Monkey Locus Coeruleus Neurons Varies With Difficulty and Performance in a Target Detection Task. J. Neurophysiol. 92, 361–371 (2004).
Article
Google Scholar
-
Bouret, S. & Sara, S. J. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 (2005).
CAS
ArticleGoogle Scholar
-
Grella, S. L. et al. Locus Coeruleus Phasic, But Not Tonic, Activation Initiates Global Remapping in a Familiar Environment. J. Neurosci. 39, 445–455 (2019).
CAS
ArticleGoogle Scholar
-
Murphy, P. R., O’Connell, R. G., O’Sullivan, M., Robertson, I. H. & Balsters, J. H. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 35, 4140–4154 (2014).
Article
Google Scholar
-
Joshi, S., Li, Y., Kalwani, R. M. & Gold, J. I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 89, 221–234 (2016).
CAS
ArticleGoogle Scholar
-
Breton-Provencher, V. & Sur, M. Active control of arousal by a locus coeruleus GABAergic circuit. Nat. Neurosci. 22, 218 (2019).
Article
Google Scholar
-
Konishi, M., Brown, K., Battaglini, L. & Smallwood, J. When attention wanders: Pupillometric signatures of fluctuations in external attention. Cognition 168, 16–26 (2017).
Article
Google Scholar
-
Beatty, J. Phasic Not Tonic Pupillary Responses Vary With Auditory Vigilance Performance. Psychophysiology 19, 167–172 (1982).
CAS
ArticleGoogle Scholar
-
Chatham, C. H., Frank, M. J. & Munakata, Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc. Natl. Acad. Sci. 106, 5529–5533 (2009).
ADS
CAS
ArticleGoogle Scholar
-
Einhäuser, W., Stout, J., Koch, C. & Carter, O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl. Acad. Sci. USA 105, 1704–1709 (2008).
ADS
ArticleGoogle Scholar
-
Knapen, T. et al. Cognitive and Ocular Factors Jointly Determine Pupil Responses under Equiluminance. PLOS ONE 11, e0155574 (2016).
Article
Google Scholar
-
Nassar, M. R. et al. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat. Neurosci. 15, 1040–1046 (2012).
CAS
ArticleGoogle Scholar
-
Privitera, C. M., Renninger, L. W., Carney, T., Klein, S. & Aguilar, M. Pupil dilation during visual target detection. J. Vis. 10, 3–3 (2010).
Article
Google Scholar
-
Karatekin, C., Couperus, J. W. & Marcus, D. J. Attention allocation in the dual-task paradigm as measured through behavioral and psychophysiological responses. Psychophysiology 41, 175–185 (2004).
Article
Google Scholar
-
Kahneman, D. Attention and Effort. (Prentice-Hall, 1973).
-
Wel, P. van der & Steenbergen, H. van. Pupil dilation as an index of effort in cognitive control tasks: A review. Psychon. Bull. Rev. 1–11, https://doi.org/10.3758/s13423-018-1432-y (2018).
-
Eldar, E., Cohen, J. D. & Niv, Y. The effects of neural gain on attention and learning. Nat. Neurosci. 16, 1146+ (2013).
CAS
ArticleGoogle Scholar
-
Mathôt, S., van der Linden, L., Grainger, J. & Vitu, F. The pupillary light response reflects eye-movement preparation. J. Exp. Psychol. Hum. Percept. Perform. 41, 28–35 (2015).
Article
Google Scholar
-
Mathôt, S., Grainger, J. & Strijkers, K. Pupillary Responses to Words That Convey a Sense of Brightness or Darkness. Psychol. Sci. 28, 1116–1124 (2017).
Article
Google Scholar
-
Binda, P., Pereverzeva, M. & Murray, S. O. Attention to Bright Surfaces Enhances the Pupillary Light Reflex. J. Neurosci. 33, 2199–2204 (2013).
CAS
ArticleGoogle Scholar
-
Papesh, M. H., Goldinger, S. D. & Hout, M. C. Memory strength and specificity revealed by pupillometry. Int. J. Psychophysiol. 83, 56–64 (2012).
Article
Google Scholar
-
Naber, M., Frässle, S., Rutishauser, U. & Einhäuser, W. Pupil size signals novelty and predicts later retrieval success for declarative memories of natural scenes. J. Vis. 13, 11–11 (2013).
Article
Google Scholar
-
Kafkas, A. & Montaldi, D. Recognition Memory Strength is Predicted by Pupillary Responses at Encoding While Fixation Patterns Distinguish Recollection from Familiarity. Q. J. Exp. Psychol. 64, 1971–1989 (2011).
Article
Google Scholar
-
Hays, W. Statistics. (Wadsworth Publishing, 1994).
-
Clark, H. H. The language-as-fixed-effect fallacy: A critique of language statistics in psychological research. J. Verbal Learn. Verbal Behav. 12, 335–359 (1973).
Article
Google Scholar
-
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. Artic. 67, 1–48 (2015).
Google Scholar
-
Kuznetsova, A., Brockhoff, P. & Christensen, R. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. Artic. 82, 1–26 (2017).
Google Scholar
-
Clayton, E. C., Rajkowski, J., Cohen, J. D. & Aston-Jones, G. Phasic Activation of Monkey Locus Ceruleus Neurons by Simple Decisions in a Forced-Choice Task. J. Neurosci. 24, 9914–9920 (2004).
CAS
ArticleGoogle Scholar
-
Chiew, K. S. & Braver, T. S. Positive Affect Versus Reward: Emotional and Motivational Influences on Cognitive Control. Front. Psychol. 2 (2011).
-
Mather, M., Clewett, D., Sakaki, M. & Harley, C. W. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 39 (2016).
-
Eldar, E., Niv, Y. & Cohen, J. D. Do You See the Forest or the Tree? Neural Gain and Breadth Versus Focus in Perceptual Processing. Psychol. Sci. 27, 1632–1643 (2016).
Article
Google Scholar
-
Warren, C. M. et al. Catecholamine-Mediated Increases in Gain Enhance the Precision of Cortical Representations. J. Neurosci. 36, 5699–5708 (2016).
CAS
ArticleGoogle Scholar
-
Lee, T.-H. et al. Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nat. Hum. Behav. 2, 356–366 (2018).
Article
Google Scholar
-
Brink, R. L., van den, Nieuwenhuis, S. & Donner, T. H. Amplification and Suppression of Distinct Brainwide Activity Patterns by Catecholamines. J. Neurosci. 38, 7476–7491 (2018).
Article
Google Scholar
-
Schwarz, L. A. & Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 25, R1051–R1056 (2015).
CAS
ArticleGoogle Scholar
-
Kebschull, J. M. et al. High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron 91, 975–987 (2016).
CAS
ArticleGoogle Scholar
-
Uematsu, A. et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 20, nn.4642 (2017).
Article
Google Scholar
-
Seo, D. & Bruchas, M. R. Polymorphic computation in locus coeruleus networks. Nat. Neurosci. 20, nn.4663 (2017).
Article
Google Scholar
-
Wang, C.-A., Blohm, G., Huang, J., Boehnke, S. E. & Munoz, D. P. Multisensory integration in orienting behavior: Pupil size, microsaccades, and saccades. Biol. Psychol. 129, 36–44 (2017).
Article
Google Scholar
-
Molen, M. W. V., der, Boomsma, D. I., Jennings, J. R. & Nieuwboer, R. T. Does the Heart Know What the Eye Sees? A Cardiac/Pupillometric Analysis of Motor Preparation and Response Execution. Psychophysiology 26, 70–80 (1989).
Article
Google Scholar
-
Reimer, J. et al. Pupil Fluctuations Track Fast Switching of Cortical States during Quiet Wakefulness. Neuron 84, 355–362 (2014).
CAS
ArticleGoogle Scholar
-
Krauzlis, R. J., Lovejoy, L. P. & Zénon, A. Superior Colliculus and Visual Spatial Attention. Annu. Rev. Neurosci. 36, 165–182 (2013).
CAS
ArticleGoogle Scholar
-
Kahneman, D. & Beatty, J. Pupil Diameter and Load on Memory. Science 154, 1583–1585 (1966).
ADS
CAS
ArticleGoogle Scholar
-
Talsma, D. & Woldorff, M. G. Selective Attention and Multisensory Integration: Multiple Phases of Effects on the Evoked Brain Activity. J. Cogn. Neurosci. 17, 1098–1114 (2005).
Article
Google Scholar
-
Mathôt, S., Siebold, A., Donk, M. & Vitu, F. Large pupils predict goal-driven eye movements. J. Exp. Psychol. Gen. 144, 513–521 (2015).
Article
Google Scholar
-
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
CAS
ArticleGoogle Scholar
-
Brainard, D. H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
CAS
ArticleGoogle Scholar
-
Pelli, D. G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
CAS
ArticleGoogle Scholar
-
Mathôt, S., Fabius, J., Van Heusden, E. & Van der Stigchel, S. Safe and sensible preprocessing and baseline correction of pupil-size data. Behav. Res. Methods 50, 94–106 (2018).
Article
Google Scholar
-
Tuszynski, J. caTools: Tools: moving window statistics, GIF, Base64, ROC AUC, etc. (2018).
